Hydrogen Fuel Cells: Clean Power from Chemistry
Dive into the science of hydrogen fuel cells—how hydrogen and oxygen combine at the anode and cathode to create electricity, the different types of fuel cells, their benefits like high efficiency and zero emissions, the challenges of hydrogen production and storage, and what the future holds for this clean energy technology.
Tapping chemical energy cleanly
In the search for low‑carbon energy sources, hydrogen fuel cells have emerged as a promising technology that can convert chemical energy directly into electricity and heat. Unlike internal‑combustion engines that burn fuel and emit pollutants, fuel cells use an electrochemical reaction between a fuel and oxygen to produce power with water or steam as the primary by‑product. They are often compared with batteries because both devices generate electricity through chemical reactions, yet there is a key difference: a battery stores a finite amount of reactants and eventually must be recharged or replaced, whereas a fuel cell can continue producing electricity for as long as fuel and oxidiser are supplied.
How a hydrogen fuel cell works
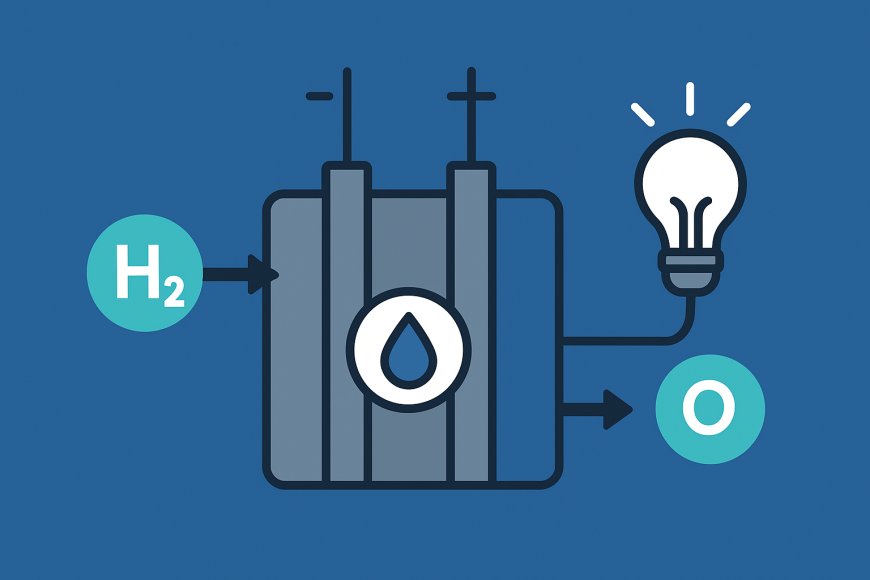
At the heart of a hydrogen fuel cell are two electrodes separated by an electrolyte. The anode is the negative electrode and the cathode is the positive electrode. In a typical proton exchange membrane (PEM) fuel cell, which is widely used in vehicles, pure hydrogen gas is fed to the anode. A catalyst at the anode splits hydrogenmolecules into protons and electrons. The protons (positively charged hydrogen ions) pass through the polymer electrolyte membrane toward the cathode. The electrons, however, cannot pass through the membrane, so they flow through an external circuit to reach the cathode. This flow of electrons provides usable electric current.
On the cathode side, oxygen from the air is supplied. The protons that have travelled through the electrolyte and the electrons that have gone through the external circuit combine with oxygen at the cathode to form water and heat. Because the only emissions are water and a small amount of heat, fuel cells offer a clean source of electricity. The overall reaction in a hydrogen–oxygen fuel cell is the reverse of electrolysis: 2H2 + O2 → 2H2O + energy. This elegant process scales from tiny fuel cells powering portable electronics to large stacks driving buses or backing up hospitals.
Types of fuel cells
Although all fuel cells share the basic operating principle of an electrochemical reaction that separates charge carriers, they vary in the materials used and the operating conditions:
- Proton exchange membrane (PEM) fuel cells use a solid polymer membrane that conducts protons. They operate at relatively low temperatures (around 80 °C) and can quickly adjust their output, making them suitable for cars, buses and backup power systems. However, they require high‑purity hydrogen and expensive platinum catalysts.
- Direct‑methanol fuel cells (DMFCs) are similar to PEM cells but use methanol as the fuel. This allows simpler fuel storage for small portable devices. The trade‑off is lower efficiency and power density than hydrogen systems.
- Alkaline fuel cells (AFCs) employ an aqueous or solid alkaline electrolyte that conducts hydroxide ions. They were originally developed for spacecraft and can achieve high efficiency. Modern versions use alkaline membranes and are being explored for lightweight applications.
- Phosphoric acid fuel cells (PAFCs) use liquid phosphoric acid as the electrolyte and operate at intermediate temperatures (around 200 °C). They are durable and have been deployed in stationary combined heat and power installations where both electricity and steamare used.
- Molten carbonate and solid oxide fuel cells (MCFCs and SOFCs) operate at higher temperatures (600 °C to 1 000 °C) using molten carbonate salts or ceramic oxides as electrolytes. The high temperature allows these cells to internally reform hydrocarbon fuels and achieve very high efficiencies. They are suited to industrial and utility‑scale power generation, often in combination with turbines.
Benefits of hydrogen fuel cells
Fuel cells offer several advantages over traditional combustion engines and even over batteries in certain contexts. They can convert the chemical energy of hydrogen into electricity with efficiencies exceeding 50 percent, and combined heat and power systems can approach total efficiencies of 80–90 percent when the waste heat is utilised. Because the reaction produces only water, fuel cell vehicles emit no carbon dioxide or tailpipe pollutants, helping to reduce air pollution and greenhouse gas emissions. Fuel cells are quiet, have few moving parts and provide steady power without the voltage drop associated with discharging batteries.
A major advantage over batteries is that a fuel cell canoperate continuously as long as it is supplied with fuel and oxidiser. Refuelling a hydrogen vehicle takes a few minutes, similar to filling a petrol tank, whereas recharging a battery can take much longer. Fuel cells can also serve as reliable backup power sources for hospitals, data centres and telecom towers, ensuring uninterrupted operation during grid outages.
Challenges and limitations
Despite their promise, fuel cells face several challenges before they can be deployed at scale. Producing hydrogen in a sustainable way is crucial; most hydrogen today comes from natural gas through steam methane reforming, which emits carbon dioxide. Cleaner pathways such as electrolysis using renewable electricity or biomass gasification are more expensive. Storing and transporting hydrogen requires high‑pressure tanks, cryogenic storage or chemical carriers, all of which add cost and complexity.
The materials used in fuel cells also present challenges. Many designs rely on platinum group metal catalysts, which are expensive and sensitive to impurities. High‑temperature cells must withstand thermal cycling and corrosion. For transportation applications, fuel cellsystems must compete with battery prices and be robust over thousands of start‑stop cycles. Building a network of hydrogen production and refuelling infrastructure is another hurdle that affects consumer adoption.
Applications and future prospects
Hydrogen fuel cells are already powering a variety of devices and vehicles. Forklifts and airport ground vehicles use PEM fuel cells because they can refuel quickly and produce no exhaust fumes. Several automakers have developed fuel cell electric vehicles (FCEVs) that offer long driving ranges and fast refuelling. Buses and trucks in some cities run on hydrogen, reducing emissions on busy routes. On the stationary side, hospitals, universities and data centres use fuel cell systems to provide reliable electricity and heat.
The future of hydrogen fuel cells depends on advances in materials science, manufacturing and hydrogen supply. Researchers are working on catalysts that use less or no platinum and membranes that are cheaper and more durable. High‑temperature fuel cells are being combined with gas turbines to achieve record efficiencies. At the same time, government incentives and private investment areaccelerating the build‑out of hydrogen production facilities and refuelling stations, especially in regions committing to decarbonisation. As renewable electricity costs decline, producing hydrogen through electrolysis becomes more viable, paving the way for fuel cells to play a significant role in transportation, industry and energy storage.
Hydrogen fuel cells offer a clean and efficient way to generate electricity by harnessing the chemical energy of hydrogen. By separating protons and electrons at the anode, guiding electrons through an external circuit and recombining them with oxygen at the cathode, fuel cells produce power and water without combustion. A range of fuel cell types has been developed to suit applications from portable electronics to heavy‑duty transport and utility‑scale power. While challenges remain in hydrogen production, cost and infrastructure, ongoing research and growing investment suggest that fuel cells could become a cornerstone of a low‑carbon energy system. Understanding the science behind fuel cells helps to appreciate both their potential and the work still needed to realise a hydrogen economy.
What's Your Reaction?