Quantum Tunneling: When Particles Defy Classical Barriers
Explore quantum tunneling, the counterintuitive quantum phenomenon that allows particles to pass through potential barriers. Learn how wave-particle duality lets electrons, protons and atoms permeate walls, why tunneling is essential for nuclear fusion and radioactivity, and how engineers harness it in tunnel diodes, flash memory and scanning tunnelling microscopes.
Introduction — a counterintuitive quantum leap
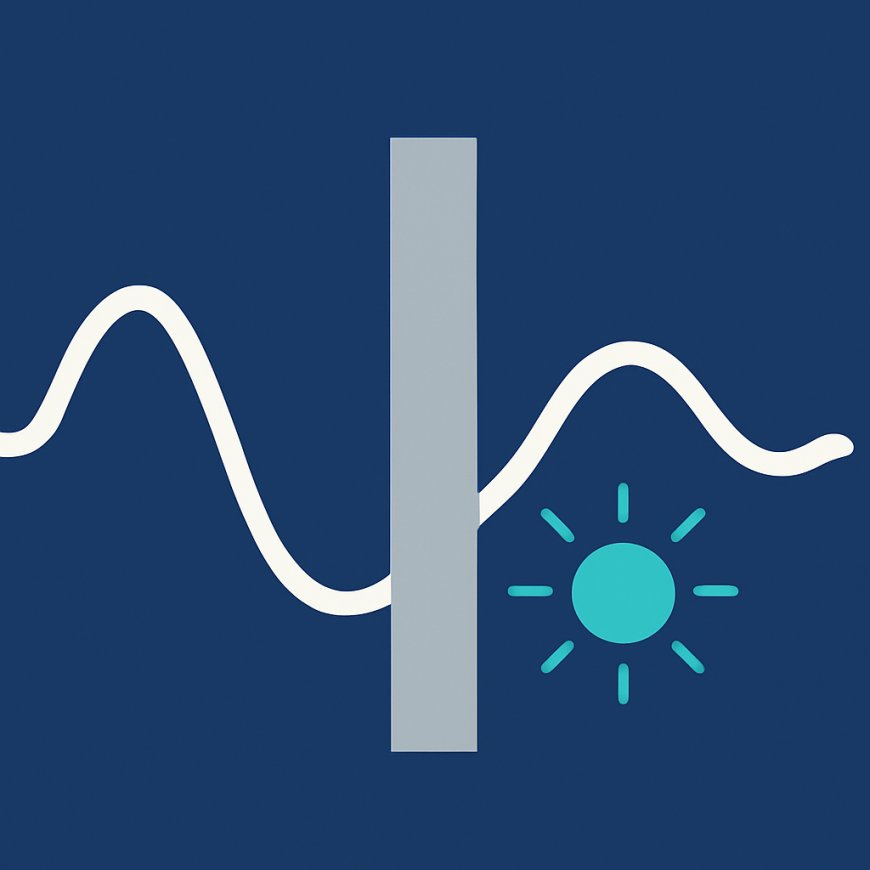
Imagine pushing a ball up a hill that is too high for it to roll over. In the classical world the ball will simply roll back down because it lacks the energy to climb the hill. Yet in the realm of quantum physics particles don’t behave like classical balls; they are also waves described by a probability amplitude. One of the strangest consequences of this wave nature is quantum tunnelling, in which a particle such as an electron or proton “passes through” a barrier that it does not have the energy to overcome. First predicted in the early 20th century and accepted a few decades later, tunnelling is now recognised as a fundamental physical process that shapes phenomena from nuclear fusion in stars to the operation of microchips.
The wave nature of matter
Quantum tunnelling arises because particles behave both as particles and as waves. Quantum mechanics describes a particle’s state via a wave function, and the square of this wave function gives the probability density of finding the particle at a given position. When a particle encounters a potential energy barrier — a region where it would need extra energy to continue — its wave function doesn’t abruptly vanish. Instead, it decays exponentially inside the barrier and continues on the other side with a reduced amplitude. The probability of the particle being found beyond the barrier is small but finite. As a result, there is a non‑zero chance that the particle will be detected on the far side even though classical physics forbids such a passage.
The likelihood of tunnelling depends on several factors. It decreases exponentially with the height and width of the barrier and increases for lighter particles. Electrons and protons can tunnel through barriers only a few nanometres thick, while heavier particles require even thinner barriers. Because tunnelling probabilities are sensitive to distance, devices exploiting the effect can detect extremely small changes in separation between surfaces.
Analogy of a ball and a hill
An everyday analogy helps illustrate why tunnelling is surprising. Consider the ball and hill scenario again. In classical mechanics, if the ball does not possess enough kinetic energy to climb over the hill, it will never appear on the other side. Quantum mechanically, however, the ball’s wave function spreads out. Part of that wave function extends into and through the barrier, giving rise to a small probability that the ball will “tunnel” through and appear on the other side. The particle doesn’t smash through the barrier; rather, the barrier fails to localise it completely. This concept challenges our classical intuition because it implies that the conservation of energy is not violated; the energy of the particle remains the same, but quantum uncertainty allows it to borrow energy temporarily to penetrate the barrier.
Tunnelling in nature
Although the probabilities are tiny on an everyday scale, tunnelling plays a pivotal role in many natural processes. In the cores of stars, positively charged nuclei need to get close enough for the strong nuclear force to overcome the electrostatic repulsion between them. At the temperatures and pressures in a star like the Sun, nuclei do not have enough kinetic energy to push through this Coulomb barrier. Quantum tunnelling allows hydrogen nuclei to overcome the barrier and fuse, releasing the energy that powers the star. Without tunnelling, nuclear fusion would require much higher temperatures and the Sun would not shine as it does. Likewise, tunnelling enables alpha decay in radioactive atoms, where an alpha particle tunnels out of the nucleus, escaping the strong nuclear potential well.
Applications in technology
Far from being an esoteric curiosity, tunnelling underpins several modern technologies. In solid‑state physics, tunnel diodes use the tunnelling of electrons through a narrow p‑n junction to achieve very fast switching and negative resistance. Flash memory stores data by allowing electrons to tunnel onto and off of floating gates. In quantum computing, qubits can be implemented in systems where tunnelling between states allows coherent superpositions. Perhaps the most direct demonstration is the scanning tunnelling microscope (STM). An STM brings an atomically sharp conductive tip to within about one nanometre of a conductive surface. When a small voltage is applied, electrons tunnel through the vacuum gap. Because the tunnelling current depends exponentially on the tip–surface separation, moving the tip up or down by a fraction of an atom’s diameter leads to a measurable change in current. By scanning the tip across the surface while keeping the current constant, the instrument maps the positions of individual atoms with unparalleled resolution.
Limits on microelectronics
Tunnelling also sets a fundamental limit on how small microelectronic devices can be. As engineers shrink transistors to nanometre scales, insulating barriers between conducting regions become so thin that electrons can tunnel through them, leading to leakage currents and power consumption. Below about one nanometre, quantum tunnelling causes devices to behave unpredictably. This is one of the reasons why semiconductor manufacturers are exploring new materials, architectures and three‑dimensional designs to continue Moore’s law.
A brief history
The theoretical basis for tunnelling emerged soon after Erwin Schrödinger formulated his equation in 1926. In 1927 Friedrich Hund studied a double‑well potential and noted that particles could penetrate the barrier between wells. Soon after, Lothar Nordheim and Ralph Fowler applied the theory to electron emission from metals, and George Gamow used it to explain alpha decay. Despite early scepticism, experiments in the 1950s and 1960s — such as Leo Esaki’s demonstration of tunnelling in semiconductor junctions — confirmed the phenomenon and led to the development of tunnel diodes. The scanning tunnelling microscope, invented in 1981 by Gerd Binnig and Heinrich Rohrer, earned its inventors the Nobel Prize for showing that tunnelling could image surfaces at atomic scales.
Quantum tunnelling reveals the profound difference between the deterministic world of classical physics and the probabilistic world of quantum mechanics. Because particles exhibit wavelike behaviour, their presence is described by a spread‑out wave function that can penetrate and even pass through barriers. The probability of tunnelling falls rapidly with barrier width and particle mass, but for electrons and atomic nuclei it is significant across nanometre distances. Tunnelling is indispensable to stellar fusion, radioactive decay, microelectronics, data storage and nanoscale imaging, yet it remains a deeply non‑intuitive phenomenon. By embracing the paradoxical nature of the quantum world, scientists and engineers have harnessed tunnelling to create technologies that once seemed impossible, and they continue to explore its applications as our control over the nanoscale grows.
What's Your Reaction?